|
30.1
Review of Atomic Structure.
Before we can study radioactivity, the spontaneous disintegration of an unstable atomic nucleus and the emission of particles orelectromagnetic radiation, we need to quickly review the basic structure of the atom.
You will recall that an atom consists of a nucleus surrounded by an electron cloud. Within the nucleus are two different types of nucleons, the protons and neutrons. These particles are held by the strong nuclear force.
- Protons and neutrons are in turn constructed of "up" and "down" quarks. Up quarks carry an electromagntic charge of +2/3 and the down quarks carry a charge of -1/3.
- The quark configuration of a proton is two up quarks and one down quark (UUD). Note that the total charge of these quarks adds up to +1.0
- The quark configuration of a neutron is one up and two down quarks (UDD). Note that the total charge of these adds up to 0.
Take a look at this graphic to review the structure described above. Take special note of the relative sizes of all the particles, and remember that this diagram IS NOT DRAWN TO SCALE!
Take some time to check out The Particle Adventure online.
Also check out the Particle Zoo
We also need to review some of the symbols we will be using in this unit. Elemental isotopes are commonly indicated in this format:
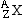
where A is the mass number, and Z is the atomic number. The mass number is the total number of nucleons and the atomic number is the number of protons in the isotope, or the number of positive charges in the nucleus.
An example is the symbol for Helium-4: 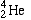
Helium-4 has two neutrons and two protons in its nucleus. This produces a mass number (A) of 4 and an atomic number (Z) of 2
Kinds of Radioactivity
As previously mentioned, radioactivity is the spontaneous disintegration of an unstable atomic nucleus and the emission of particles and/or electromagnetic radiation.
Pierre and Marie Curie investigated uranium ores using chemical separation. They discovered that pitchblende and chalcocite, naturally occurring ores, were highly radioactive due to the presence of plutonium and radium.
All naturally occurring elements with atomic numbers greater than 83, as well as some isotopes of lighter elements, are radioactive. Radioactive isotopes are also known as radioisotopes. There are several ways in which radiation is measured. Check this link for the class handout.
Based on later work by Rutherford, Soddy, Villard, and others, three different types of radiation were identified.
- Alpha particles(
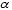 ) are helium nuclei, containing two protons and two neutrons. ) are helium nuclei, containing two protons and two neutrons.
- They are deflected slightly in an electric of magnetic field (see graphic below).
- Their penetrating power is very low, being stoppable by a thin sheet of aluminum or paper.
- The loss of an alpha particle from the nucleus results in a decrease in the atomic mass of the nucleus. The end result is a new element with a mass number 4 less than its parent, and an atomic number 2 less than its parent. Such a change, resulting in a new element, is called a transmutation. For example, if Uranium -238 (
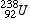 ) undergoes an alpha decay, it becomes Thorium-234 ( ) undergoes an alpha decay, it becomes Thorium-234 (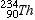 ). ).
- Beta particles(
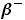 ) are electrons capable of travelling at speeds approaching the speed of light. ) are electrons capable of travelling at speeds approaching the speed of light.
- Their low mass allows them to be deflected greatly in an electric or magnetic field, in the opposite direction as the deflection of alpha particles (see graphic below)
- Their high speed gives them greater penetrating power than alpha particles. Some beta particles can penetrate several centimeters of aluminum.
- In beta decay, neutrons re-organize into a proton and a beta particle is ejected. The result of this is a transmutation into a new element with the same mass number as the parent, but an atomic number that is one higher.. due to the appearance of the new proton. For example, Th-234 beta decays into Protactinium-234 (
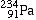 ) )
- Alpha particle emissions and beta particle emissions change the composition of the nucleus.
- Gamma (
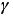 ) rays are high energy electromagnetic radiation with short wavelengths. ) rays are high energy electromagnetic radiation with short wavelengths.
- Gamma rays, unlike alpha and beta particles, do not change the composition of the nuclide.
- They have the highest penetrating power, being able to penetrate at least 30 centimetres of lead.
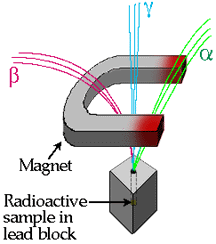
All radioactive nuclides have the following common characteristics:
- Their radiations
- affect the emulsion of photographic film
- ionize surrounding air molecules
- make certain compounds fluoresce,
- and have certain special biological effects.
- They undergo radioactive decay.
Nuclear Reactions and equations.
When a radioisotope undergoes a transmutation, a nuclear reaction has taken place. Nuclear reactions can be written if a format similar to other chemical reactions. We need to look at a few symbols for subatomic particles first, so that we can easily write the equations:
|
Particle
|
Symbol
|
| Proton |
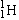 or or 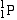 |
| Neutron |
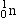 |
| alpha particle |
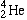 or or 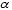 |
| beta particle or electron |
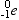 or or 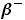 |
| positron |
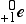 or or 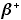 |
| neutrino |
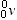 |
| antineutrino |
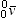 |
| gamma (photon) |
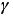 |
An alpha decay can be written as follows:
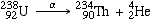
- U-238 alpha-decays into Thorium-234 plus an alpha particle
- Note that mass numbers on the left equal the mass numbers on the right. While atomic mass number is conserved, total mass IS NOT. There is less mass on the right side of the equation. This is accounted for by the direct conversion of mass to energy according to Einstein's formula, E = mC2 , and the resulting energy is manifest as kinetic energy of the alpha particle. We will discuss this missing mass, called the mass defect, at a later time.
A beta decay can be written as follows:
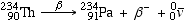
- In beta decay, a down quark in a neutron has converted into an up quark, converting the neutron into a proton, plus a W- boson which then decays into an electron and an antineutrino.
- Thorium-234 beta-decays into Protactinium-234 plus a beta particle and an antineutrino
- Note again that the mass numbers are conserved on both sides of the equation, and again while atomic mass number is conserved, actual mass IS NOT. There is a mass defect on the right side, and is manifest primarily as kinetic energy of the beta particle and the neutrino. In fact, the existence of the neutrino was originally "invented" to help account for the mass defect in this type of reaction.
Half Life.
The half life of a radionuclide is the amount of time after which one half of an original sample is expected to have decayed into something else.
For example, the half life of U-238 is about 4.5 billion years.
- That means that if you started with 1.0 kg. of U-238 and hung around for 4.5 billion years, about 0.5 kg would still be U-238 and the other 0.5 would be other elements.
- U-238 alpha-decays into Thorium-234, but this doesn't mean that after 4.5 billion years you would have 0.5 kg of remaining U-238 and 0.5 kg of Th-234.
- Th-234 has it's own half life.. about 24 days, and after 4.5 billion years most of the Th-234 would have also decayed into other materials, and so on. So as soon as one atom of U-238 decays.. you have some Thorium, which could decay at any time into Protactinium, etc.
- In fact, the entire decay series for U-238 involves 14 steps, with the ultimate final nuclide being Pb-206, which is not radioactive and thus has no half-life at all.. it's permanent.
- Also... you don't have to wait around for 4.5 billion years, and then all of a sudden 50% of the sample converts to Th-234 all at once. NO.. the process goes on all the time.
- Note that we currently place the age of the Earth at about 4.5 billion years.. very close to the half life of U-238. So... of all the U-238 that was present at the time of the Earth's inception, only about one half of it still remains!
There is a way to determine how much of a given sample would be still in its orginal nuclear form after a certain time period has elapsed. For example.. if you start with 1.0 kg of Uranium-238, how much would be left after 3.15 x 1010 years? Here's how you find out..
- 1. Figure out how many half lives have elapsed. Divide the elapsed time by the half life. In this case.. 3.15 x 1010 years/4.5 x 109 years = 7 half lives.
- 2. Then, use the formula
- mass remaining = original mass x (1/2)N where N is the number of half lives from step 1.
- In this example, our mass remaining = 1.0 kg x (1/2)7 = 0.0078 kg. (less than 1%)
Knowledge of half-lives is used in some archeological techniques known as radiometric dating.
Radiometric Dating is a process of judging the age of a material, based upon the amount of a particular parent radioisotope that is found in the sample, compared to how much is expected to be there. Carbon-14 (half-life = 5.74 x 103) is one radioisotope that is used for this.
- Carbon 14 (C-14) is a relatively rare isotope of carbon, accounting for only about10-8 percent of all the atmospheric carbon. C-14 is continually produced in the earth'supper atmosphere as a result of neutron capture by N-14 nuclei.
- The neutrons are the product of cosmic ray interaction with air molecules. Due to atmospheric mixing there is a consistent ratio of C-14 to all other isotopes of carbon in the atmosphere.
- Living organisms are constantly exchanging carbon. In photosynthesis, CO2 is removed from the atmosphere and fixed into carbohydrates. Nonphotosynthetic organisms acquire carbon from photosynthetic organisms and release CO2 during respiration.
- Thus, the ratio of C-14 to nonradioactive carbonisotopes in living organisms is the same as that in the atmosphere.
- When an organism dies and is removed from contact with the atmosphere no new carbon 14 is acquired.
- Over time, the amount of C-14 will be less than the atmospheric level.
- If the sample has been exposed to atmospheric C-14, correction factors can be applied allowing successful dating to performed.
- A major assumption of this method is that the cosmic ray flux has been constantover time. All current observations indicate this to be a correct assumption at least for the past 5,000 to 10,000 years.
- There are some other assumptions in this process:
- 1.Numbers of parent and daughter atoms must be quantifiably measurable
- 2.Parent element must decay rapidly enough to produce measurable amounts of the daughter element, but measurable amounts of the parent element must also be present in the sample
- 3.Little orno daughter element must have been present in the sample when it originated
- 4.The sample must have been kept in a chemically close system with respect to the parent and daughter elements through out its history
- How long would it take for C-14 to decay to the point where less than 1% of it's original sample remained? After this point, the accuracy of the dating procedure would be in question.
- More on radiometric dating from the USGS
Homework: read chapter 30.1 Problem set#1: Ch 30, probs 18-27
Lab: Half life
Activity: Decay chain of Uranium. Get the HTML version of the activity
Self Learning: the Particle Adventure. Download the .PDF version of the quiz and handout
|
|
